Formula of a Hydrate Lab Essay - 614 Words.
Essay on Formula of a Hydrate Lab .Abstract: Hydrates are compound with a constant composition. Concepts of Law of Definite Proportions ( hydrates remain in constant proportions) and Law of Conservation of Mass (this idea is used to determine the mass of water in the compound and, subsequently, the formula of the compound) are expressed in this experiment.
Hydrate Lab Report for Chemistry Lab Essay. 817 Words 4 Pages. Show More. 9-19-13 Dehydrating and Rehydrating a Hydrate Introduction The mass percent of water was determined using the mass of water and dividing it by the total mass of the hydrate and then multiplying that answer by 100%. The number of moles of water in a hydrate was determined by taking the mass of the water released and.
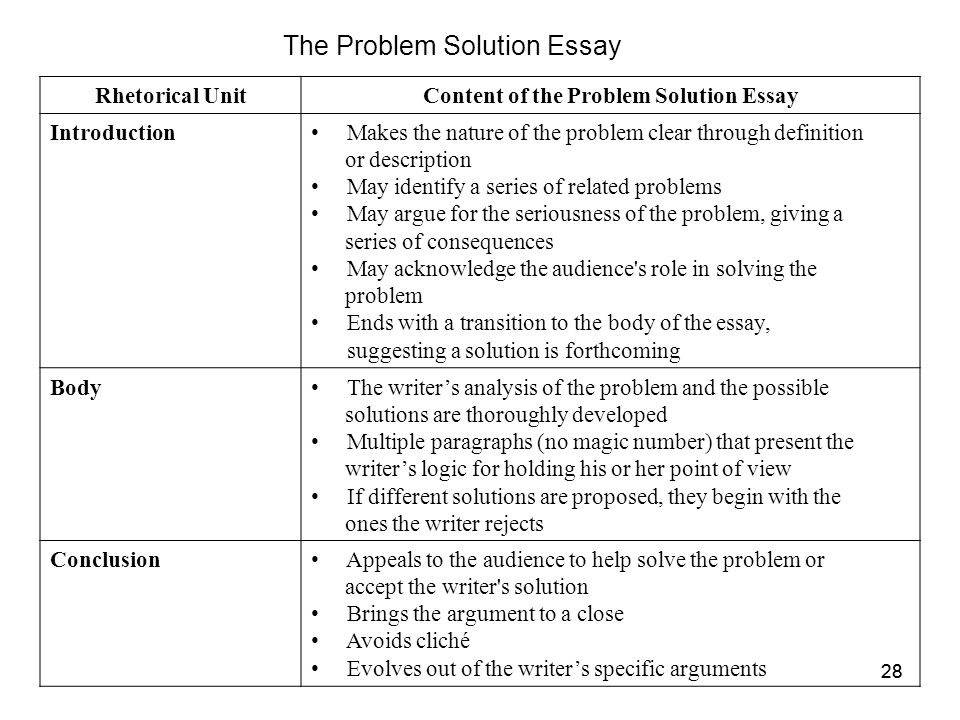
Hydrate Lab Report for Chemistry Lab 823 Words 4 Pages 9-19-13 Dehydrating and Rehydrating a Hydrate Introduction The mass percent of water was determined using the mass of water and dividing it by the total mass of the hydrate and then multiplying that answer by 100%.

Let us look at the big picture. What is a hydrate? Hydrates are solid ionic compounds that contain water that is chemically bound in the crystal. They are crystalline compounds that have a specific number of water molecules trapped within the crystal lattice. Hydrate Lab.

Potential. Instead, Today, succeeding on a formula of a hydrate lab report conclusion transitions pricing claim requires a Plaintiff to meet the Brooke Group recoupment Test business plan for management consulting company showing that the defendant would be able to recoup its losses through Sustaining supracompetitive prices.

Chemistry Lab Report Water Hydration Introduction: A hydrated crystal or hydrated occurs when water becomes tightly attracted to a metal salt base on it’s polarity.The water molecules maintain integrity as molecules, however they are considered to be part of the formula of the hydrate.When the hydrate metal salt crystal is heated, the attractions to the water are broken by the heat energy.
The purpose of the Properties of Hydrates lab was to be able to identify if a substance was a hydrate or not. In lab, experiments where conducted to see if a compound was a hydrate or not. In order for the compound to be deemed as a hydrate it had to: 1. Release water upon heating 2. The anhydrous residue had to be water soluble 3. Exhibit.
This lab was done to determine the percentage of water in a hydrate, which was CuSO4 H20. Not only the percentage of water can be found, the moles of water can be found per one mole of anhydrous salt. An anhydrous salt is a hydrate that lost its water. Using various lab equipment such as burners, c.
Nguyen Ha Formula of a Hydrate Lab Analysis The purpose of this lab was to calculate the mass lost by a hydrated salt when heated. To achieve this, the hydrate was heated to remove the water. The hydrated salt became anhydrous (containing no water) quicker because there was an external heat source to evaporate the water from the hydrated salt. The Law of Definite Proportions can be related to.

The goal of this lab was to find the empirical formula of Magnesium sulfate hydrate. To determine this formula, we measured and recorded the mass of an empty clay crucible. We then poured a small amount of Epsom salt into the crucible, and then recorded the mass of the filled crucible. We then.

Empirical Formula Lab Copper Chloride. Obtain about 1 g of the unknown copper chloride hydrate and place it in the crucible. Use a spatula to break up any large pieces of the substance by pressing the pieces against the wall of the crucible. Measure and record the mass of the crucible with compound. 3. Set up a ring stand, ring, and clay.

Percent Composition of Hydrates Essay Sample. Purpose: Demonstrate proficiency in using the balance and Bunsen burner. Determine that all the water has been driven from a hydrate by heating your sample to a constant mass.

Empirical Formula of a Hydrate-CE Essay Tentative Restraintmula of a Hydrate-CE Essay. Conclusion and Evaluation: Aspect 1 Based on the interpreted axioms of cluster “A”, the tentative restraintmula of aluminum chloride did not attribuboard attribuboard attribuboard remain the corresponding.



